Diffusion and Effusion play an essential role in understanding various scientific and natural phenomena and in this article we’ll delve into their fascinating world – their definitions, potential influences on them, application in daily life as well as through research studies.
What is Diffusion?
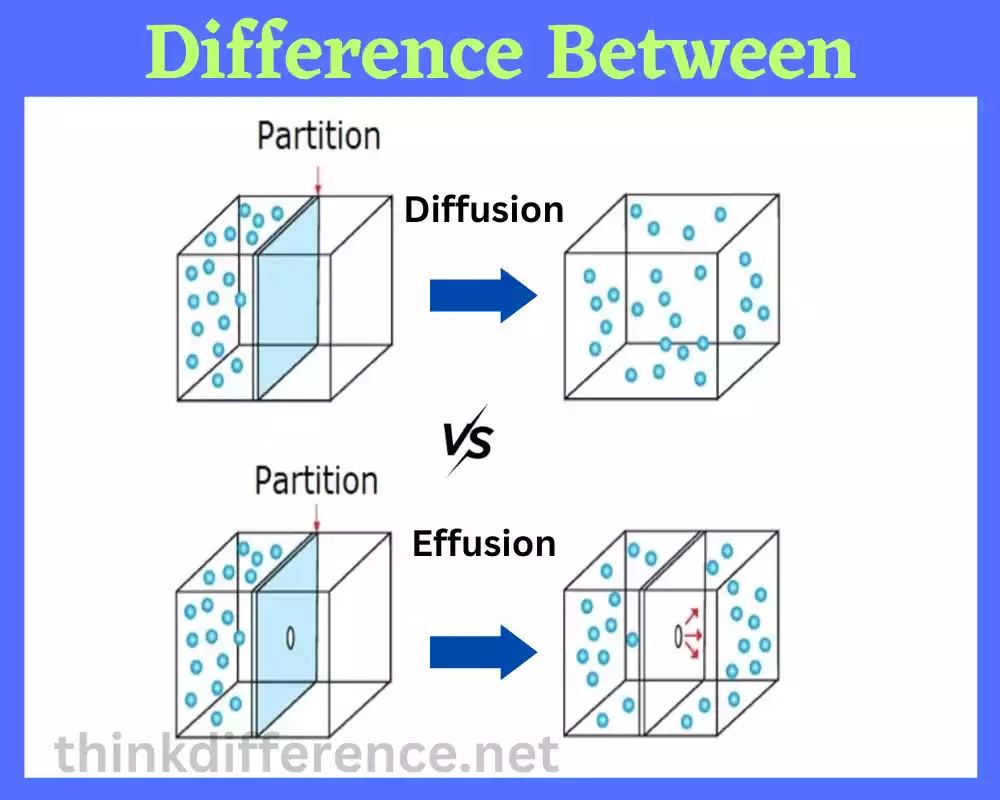
Diffusion refers to the process by which molecules, particles or ions travel from areas with increased concentration to ones with decreased concentration, often without preplanning but driven by natural tendencies for particles to expand and reach equilibrium. It’s often an unintentional phenomenon driven by natural forces of expansion of particle populations within different environments.

Under diffusion, particles move at random and collide, leading to an overall shift from areas with high concentration to those with lower concentration, gradually reaching equilibrium point until all concentrations reach uniform throughout the system and reach uniformity across its entirety.
Diffusion occurs across many types of matter, from liquids and gases to solids. Gas particles have much greater freedom to move around due to diffusion.
According to experts, diffusion occurs due to multiple factors including temperature, concentration gradient and surface area. Higher temperatures tend to speed up diffusion by increasing particle kinetic energy while an increased concentration gradient and larger surface area can speed it up further.
Diffusion is a crucial aspect of industrial and natural processes alike. For instance, diffusion enables exchanges of gases within our respiratory systems; absorption of nutrition into cells. Dispersion of environmental pollutants from chemical reactions and mixing chemicals is possible due to diffusion. Recognizing diffusion’s importance can benefit many fields like biology, chemistry, physical science and environmental sciences.
Factors Affecting Diffusion
Several factors influence the rate and extent of diffusion. These factors include:
- Temperature: Temperature has a significant impact on diffusion. As temperatures increase, particle kinetic energy increases causing increased molecular movement and speedier diffusion. Higher temperatures result in more frequent and energetic collisions between particles, promoting their movement and dispersion.
- Concentration gradient: The concentration gradient or the difference in concentration between two regions, is a driving force for diffusion. More varied the concentration, greater will be its diffusion speed. A steeper concentration gradient provides a stronger driving force, causing particles to move more rapidly from areas of high concentration to areas of low concentration.
- Molecular size and mass: The size and mass of particles affect their diffusion rate. Smaller and lighter particles diffuse more quickly than larger and heavier particles. This is because smaller particles experience less resistance from the surrounding medium and have higher average velocities, allowing them to move more rapidly through the medium.
- Medium or medium properties: The nature of the medium through which diffusion occurs can influence the diffusion rate. Viscosity density, intermolecular forces in a medium and other factors such as viscosity may all impact how quickly particles diffuse into their surroundings. In a less viscous or less dense medium, particles can move more freely, promoting faster diffusion.
- Surface area: The surface area available for diffusion affects the rate of diffusion. A larger surface area provides more space for particles to interact and move, increasing the rate of diffusion. For example, finely divided solids or porous materials have higher surface areas, leading to enhanced diffusion rates compared to larger solid objects.
- Distance or thickness: The distance or thickness of the medium through which diffusion occurs can impact the rate of diffusion. A shorter distance or thinner medium allows particles to travel a shorter path, reducing the time required for diffusion. In contrast, a longer distance or thicker medium slows down diffusion.
Noting the interrelation between all these components is crucial in order to properly understanding their influence on diffusion mechanisms, their efficiency and speed of performance.
Applications of Diffusion
Diffusion has numerous applications across various fields. Some notable applications of diffusion include:
- Biological Systems: Diffusion plays a critical role in biological systems. Diffusion aids the movement of ions and molecules across cell membranes, helping the absorption of nutrients, release of waste products, exchanging of gases during respiration as well as exchanging of gas when respiration takes place. Diffusion also plays an integral part in processes like osmosis which involves water movement through semipermeable barriers.
- Pharmaceutical Industry: In the pharmaceutical industry, diffusion is utilized for drug delivery. Controlled-release drug formulations are designed to release drugs gradually through diffusion, ensuring a sustained and controlled release of medication into the body over an extended period. This approach allows for improved therapeutic outcomes and reduces the frequency of drug administration.
- Environmental Science: Diffusion plays a crucial role in various environmental processes. It aids in the dispersion of pollutants in air and water, allowing for their gradual dilution and dispersal. Diffusion is also involved in the exchange of gases between the atmosphere and bodies of water, influencing factors such as oxygen levels and pH balance in aquatic ecosystems.
- Materials Science: Diffusion is vital in materials science for processes such as heat treatment and alloying. In heat treatment, diffusion allows for the controlled movement of atoms within a solid, leading to changes in its microstructure and properties. Diffusion bonding involves the joining of materials through the diffusion of atoms across the interface, resulting in a strong bond.
- Chemical Reactions: Diffusion plays a role in chemical reactions, particularly in heterogeneous systems. It facilitates the movement of reactant molecules to the reaction sites, allowing for effective collision and reaction. Diffusion also aids in the transport of reactants and products within catalysts, enhancing their efficiency.
- Membrane Technology: Diffusion is a fundamental mechanism in various membrane-based technologies. Processes such as reverse osmosis, dialysis and gas separation rely on the selective diffusion of solutes or gases across semipermeable membranes to achieve separation and purification.
- Food and Beverage Industry: Diffusion is employed in the food and beverage industry for processes like flavor extraction, where the aroma compounds diffuse from the food matrix into a solvent or carrier. Diffusion is also used in processes such as fermentation, where microorganisms diffuse into food substrates for fermentation and production of various food products.
These are just a few examples of the diverse applications of diffusion in various fields. Understanding and controlling diffusion processes are crucial for advancing technology, improving healthcare and addressing environmental challenges.
What is Effusion?
Effusion refers to the process in which a gas escapes or flows through a small opening or a porous barrier into a region of lower pressure. It involves the movement of gas particles from a container or confined space to an area with lower gas pressure.
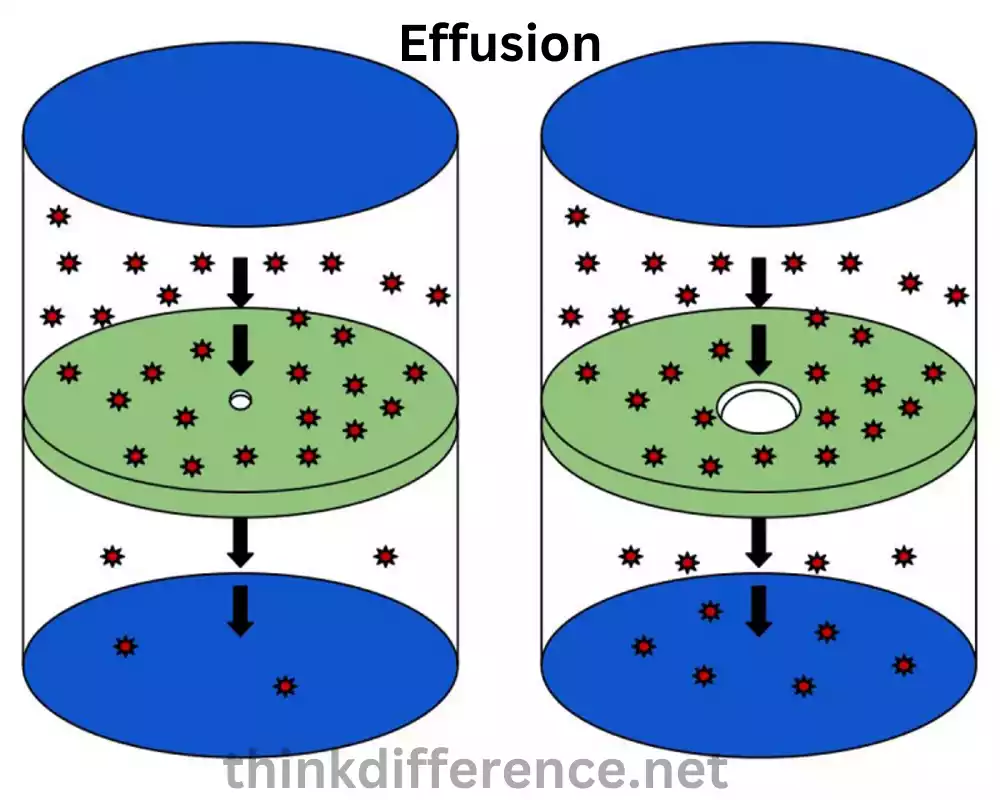
In effusion, gas particles near the opening or barrier gain enough kinetic energy to overcome the attractive forces within the gas and pass through the opening. This escape of gas particles occurs due to the pressure difference between the inside and outside of the container or system. The higher pressure inside pushes the gas particles toward the opening and they flow out into the region of lower pressure.
Effusion is influenced by factors such as the size of the opening or barrier, the pressure difference and the properties of the gas molecules, such as molecular mass and temperature. Lighter gas molecules effuse more rapidly than heavier ones, smaller openings restrict the flow and slow down effusion and larger pressure differences lead to faster effusion rates.
Effusion has practical applications in various fields. It is used in gas separation processes, such as the separation of isotopes, and in leak detection systems. Effusion is also relevant in vacuum technology, as it contributes to the removal of gas molecules from a chamber to create and maintain a vacuum. Understanding and studying effusion aids in the development of scientific theories and models related to gas behavior and transport phenomena.
Factors Affecting Effusion
Rate of effusion or gas flow through an opening are determined by various variables, some key ones include:
- Molecular Mass: The molecular mass of the gas affects the rate of effusion. Graham’s Law of Effusion suggests that effusion rate depends on a ratio between square roots to molecular weight of gas being released and effusion. Heavier gas molecules have lower average velocities and, therefore, effuse more slowly compared to lighter gas molecules. This relationship implies that gases with lighter molecules effuse more rapidly through a given opening.
- Pressure Difference: The pressure difference between the inside and outside of the container or system affects the rate of effusion. A larger pressure difference drives a faster flow of gas through the opening. The greater the pressure difference, the more significant the force pushing the gas molecules out, resulting in a higher effusion rate.
- Temperature: Temperature influences the kinetic energy of gas molecules, which in turn affects their effusion rate. Higher temperatures result in greater molecular motion and higher average velocities of gas particles. As a result, at higher temperatures, gas molecules have increased energy to overcome the attractive forces and effuse more rapidly.
- Size and Geometry of the Opening: The size and geometry of the opening through which effusion occurs impact the rate of effusion. A smaller opening restricts the flow of gas particles, leading to a slower effusion rate. A larger opening allows for a more substantial flow of gas particles, resulting in a faster effusion rate.
- Nature of the Gas: Viscosity and intermolecular forces, among other physical characteristics of gasses, may play an influential role in how quickly their effusion rate occurs. Gases with lower viscosities or weaker intermolecular forces often have greater effusion rates as opposed to those characterized by greater viscosity or stronger forces between molecules.
- Mean Free Path: The mean free path, which is the average distance a gas molecule travels between collisions with other gas molecules, also affects effusion. A longer mean free path allows gas molecules to move more freely and reach the opening more frequently, resulting in a higher effusion rate.
Recognise that these elements often interact, with their interactions determining the rate of effusion. The conditions and specific setup of the system can also impact effusion.
Applications of Effusion
Effusion has various applications in different fields. Some notable applications of effusion include:
- Gas Separation: Effusion is employed in gas separation processes. In the production of isotopically enriched gases, such as separating isotopes of uranium for nuclear fuel, effusion is utilized to achieve separation based on the different effusion rates of gas isotopes. This approach allows the extraction and purification of isotopes used for medical, scientific and industrial uses.
- Leak Detection: Effusion is useful in leak detection and monitoring systems. By measuring the rate of effusion through small openings or barriers, it is possible to detect leaks in gas containers, pipelines or sealed systems. Monitoring the effusion rate helps identify and locate leaks, ensuring safety, preventing loss and maintaining the integrity of gas containment systems.
- Vacuum Technology: Effusion plays a crucial role in vacuum systems and technologies. In processes like vacuum pumping, effusion assists in removing gas molecules from a chamber to create and maintain a vacuum. Effusion rates are monitored and controlled to achieve the desired vacuum levels in applications such as semiconductor manufacturing, vacuum deposition and analytical instrumentation.
- Mass Spectrometry: Effusion is utilized in mass spectrometry, a powerful analytical technique. Mass spectrometers separate and analyze ions based on their mass-to-charge ratios. Effusion is involved in the ionization and acceleration of gas molecules, allowing them to enter the mass spectrometer and undergo further separation and detection.
- Gas Flow Control: Understanding effusion is essential for gas flow control in various processes. Effusion rates can be manipulated by adjusting factors such as pressure differentials, opening sizes and temperature, enabling precise control over the flow of gases in applications like gas chromatography, chemical reactors and industrial gas supply systems.
- Fundamental Gas Behavior Studies: Effusion serves as a basis for studying the fundamental behavior of gases and validating theoretical models. By measuring effusion rates and comparing them with predicted values, scientists can assess and refine theories related to gas dynamics, transport phenomena and intermolecular interactions.
Effusion has practical implications in diverse industries, including chemistry, physics, materials science, semiconductor manufacturing and environmental monitoring. Its applications range from separation and purification processes to leak detection and vacuum technology, contributing to advancements in various scientific and technological fields.
Comparison Between Diffusion and Effusion
Diffusion and effusion are both processes that involve the movement of particles, specifically in gases. There are key differences between diffusion and effusion. Here’s an analysis of both methods:
1. Definition:
- Diffusion: Diffusion refers to the unintentional movement of particles from one area with high concentration to an area with lower concentration, leading to their spread or mixing together and eventually mixing of various substances together.
- Effusion: Effusion refers to the process by which gas particles escape through small openings and barriers at lower pressure to an area with lower atmospheric pressure.
2. Mechanism:
- Diffusion: Diffusion occurs due to the random motion of particles, leading to their gradual mixing or spreading. It is driven by the concentration gradient and the collisions between particles.
- Effusion: Effusion involves the flow of gas particles through a small opening or barrier. Control is determined by comparing pressure differences on either end of the unit.
3. Driving Force:
- Diffusion: The driving force for diffusion is the concentration gradient. Particles move from areas of higher concentration to areas of lower concentration to achieve equilibrium.
- Effusion: The driving force for effusion is the pressure difference. Gas particles escape or flow through the opening from regions of higher pressure to regions of lower pressure.
4. Factors Affecting Rate:
- Diffusion: Diffusion rate can be affected by variables like temperatures, concentration gradient and surface area. Higher temperatures, steeper concentration gradients and larger surface areas generally result in faster diffusion rates.
- Effusion: The rate of effusion is influenced by factors such as molecular mass and the size of the opening. Lighter gas molecules effuse more quickly than heavier ones and larger openings allow for faster effusion.
5. Examples:
- Diffusion: Examples of diffusion include the spreading of perfume in a room. The mixing of gases in the air and the exchange of gases in biological processes like respiration.
- Effusion: Examples of effusion include gas escaping from a balloon, the release of gas from a pressurized container and the flow of gas through a small hole.
Diffusion and effusion are related processes involving the movement of gas particles, but they differ in terms of their mechanisms, driving forces and factors affecting their rates. Diffusion occurs due to the random motion of particles driven by concentration gradients, while effusion involves the flow of gas particles through small openings driven by pressure differences. Understanding these differences is important in various scientific, industrial and technological applications.
Similarities Between Diffusion and Effusion
Diffusion and effusion share some similarities as they both involve the movement of particles, particularly in gases. Here are the similarities between diffusion and effusion:
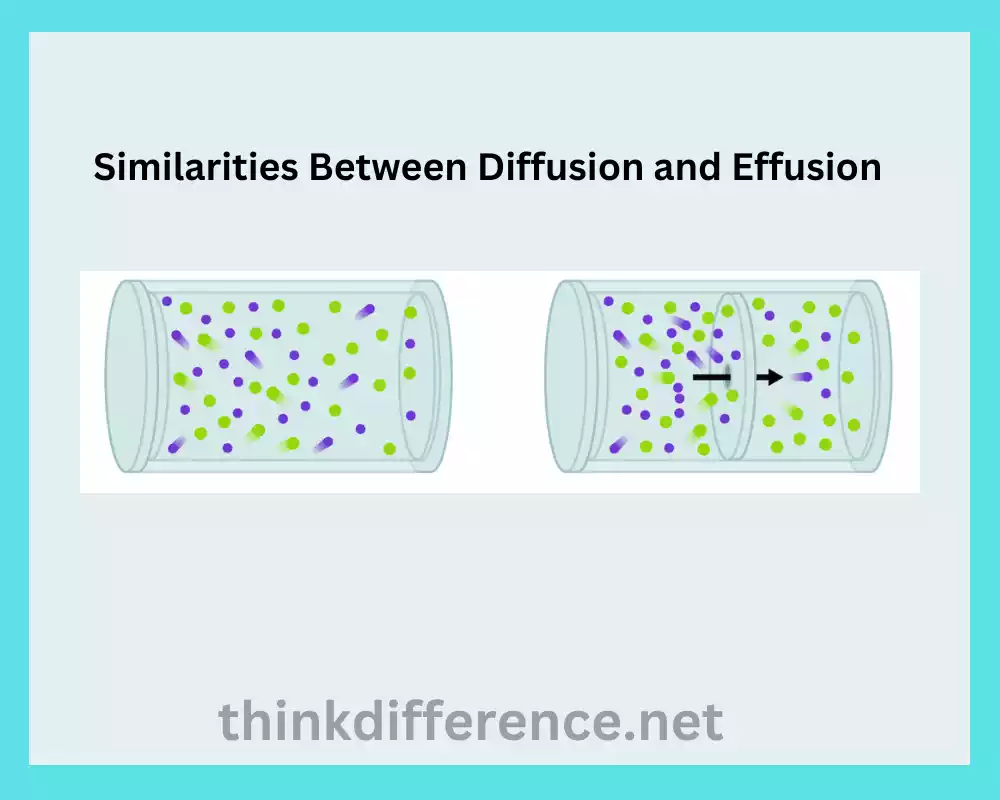
- Particle Movement: Both diffusion and effusion involve the movement of particles, such as gas molecules, from one region to another. Particles are transferred from areas with higher pressure or concentration to those with reduced levels.
- Spontaneous Process: Diffusion and effusion are spontaneous processes that occur naturally without requiring external forces or intervention. They occur due to the inherent kinetic energy and random motion of particles.
- Equalization of Concentration/Pressure: Both diffusion and effusion lead to the equalization or homogenization of concentration or pressure. In diffusion, particles move to areas of lower concentration, resulting in a more uniform distribution of the substance. In effusion, gas particles escape from regions of higher pressure to regions of lower pressure, leading to pressure equalization.
- Influenced by Molecular Properties: The rates of diffusion and effusion are influenced by the molecular properties of the particles involved. Factors such as molecular mass, temperature and intermolecular forces affect the rates at which particles diffuse or effuse.
- Follow Physical Laws: Both diffusion and effusion follow physical laws and principles. For example, Graham’s law of diffusion describes the relationship between the rates of diffusion of different gases, while Boyle’s law and Charles’s law apply to the behavior of gases during effusion.
- Important in Gas Transport: Diffusion and effusion are significant in the transport of gases. Diffusion allows biological systems to exchange gases among themselves, for instance the exchange of carbon dioxide within our lungs. Effusion plays a role in processes like gas separation, vacuum technology and gas flow control.
While diffusion and effusion share these similarities, it is important to note that they are distinct processes with different mechanisms, driving forces and factors affecting their rates. Diffusion occurs in open systems and involves the mixing or spreading of substances, while effusion occurs through small openings or barriers and involves the flow of gas particles.
Diffusion and Effusion in Daily Life
Diffusion and effusion are phenomena that occur in our daily lives, often without us being consciously aware of them. Here are some examples of diffusion and effusion in everyday situations:
Diffusion:
- Perfume: As soon as you spray perfume in an area, its fragrance disseminates throughout and you can smell it even from faraway.
- Cooking Odors: When you cook something aromatic, such as onions or garlic, the smell diffuses throughout the house, reaching different rooms and areas.
- Air Fresheners: Air freshening devices such as diffusers or aerosol sprays release pleasant scents into the atmosphere, leaving an aromatic backdrop. These fragrances diffuse, spreading a pleasant smell throughout the room.
- Spreading of Spices: When you sprinkle spices, like cinnamon or nutmeg, onto food or into a hot beverage, the flavor compounds diffuse and distribute evenly, enhancing the taste.
- Foggy Windows: On a cold day, when you breathe on a glass surface like a window, the moisture from your breath condenses and forms fog. The fog disappears as water vapor diffuses into the surrounding air.
Effusion:
- Gas Leakage: When there is a gas leak, such as from a stove or a gas cylinder, the gas particles effuse through any available openings, causing a noticeable smell or sound.
- Balloon Deflation: When you release the opening of an inflated balloon, the gas particles inside effuse out through the small opening, causing the balloon to deflate gradually.
- Tire Puncture: If you have a punctured tire, the air inside the tire effuses out through the small hole, leading to a gradual loss of tire pressure.
- Sprinkler System: In a sprinkler system, water flows through small openings or nozzles, effusing into the air and creating a fine mist that falls onto plants or lawns.
- Inhalation of Medicinal Vapors: When using an inhaler or a nebulizer for respiratory medication, the medicine is released as a mist or vapor. The tiny particles effuse into the airways, allowing for absorption and therapeutic effects.
These examples illustrate how diffusion and effusion play a role in everyday experiences, whether it’s the spread of scents, the release of gases or the distribution of substances in various contexts.
Importance of Diffusion and Effusion in Science
Diffusion and effusion are of great importance in the field of science and have numerous applications across various disciplines. Here are some reasons why diffusion and effusion are significant in science:
- Understanding Molecular Movement: Diffusion and effusion provide insights into the behavior and movement of molecules. They help scientists comprehend how particles spread, mix, and interact with their surroundings, contributing to our understanding of molecular dynamics and transport phenomena.
- Gas Behavior and Kinetic Theory: Diffusion and effusion experiments provide evidence and support for the kinetic theory of gases. These processes align with the assumptions of the theory, such as the random motion of gas particles and their collisions. Studying diffusion and effusion allows scientists to test and validate theoretical models that describe the behavior of gases.
- Measurement of Properties: Diffusion and effusion are used to measure and determine various properties of substances. For example, diffusion coefficients are essential in fields like material science, chemical engineering and environmental studies. By measuring diffusion rates and studying the factors that influence them, scientists can extract valuable information about the properties and behavior of substances.
- Gas Separation Techniques: Diffusion plays a crucial role in separation processes, particularly in gas separation techniques. Methods like gas chromatography, membrane separation and distillation rely on the differential rates of diffusion to separate and analyze mixtures of gases. These techniques have widespread applications in chemical analysis, environmental monitoring, and industrial processes.
- Pharmaceutical Science and Drug Delivery: Diffusion is vital in pharmaceutical science for drug delivery systems. Understanding the diffusion of drugs through various biological barriers, such as skin or cell membranes, helps optimize drug formulations, control release rates and enhance therapeutic efficacy.
- Environmental Studies: Diffusion is an integral component in the transportation and dispersion of pollutants within an environment, impacting particle and gas polluted with pollutants throughout air, water and soil environments. Understanding diffusion processes helps scientists model and predict the dispersion of contaminants, contributing to environmental risk assessment and pollution control strategies.
- Material Science and Engineering: Diffusion is a fundamental process in material science and engineering. It influences phenomena like heat transfer, corrosion and phase transformations in materials. By studying diffusion, scientists can design and engineer materials with desired properties, optimize manufacturing processes and improve the performance of various devices and structures.
Diffusion and effusion are essential concepts in science, enabling us to understand molecular movement, study gas behavior, measure properties, develop separation techniques, enhance drug delivery systems, analyze environmental processes and advance materials science and engineering. Their significance extends across multiple scientific disciplines, making them valuable tools for research, experimentation and technological advancements.
Conclusion
Diffusion and Effusion are essential processes that govern the movement of gases. Diffusion refers to the spontaneous mixing of gas molecules, while effusion is the escape of gas molecules through a small opening. These phenomena have a significant impact on our daily lives, chemistry, nature, and various industries.
Understanding the principles of diffusion and effusion allows us to comprehend many natural and artificial processes, leading to further advancements in science and technology.