Myasthenia Gravis and Lambert Eaton Myasthenic Syndrome are both neuromuscular disorders characterized by muscle weakness and fatigue, albeit with distinct etiologies. MG is an autoimmune condition where antibodies target the acetylcholine receptors at the neuromuscular junction, hindering efficient muscle contraction. This typically manifests as drooping eyelids, blurred vision, difficulty swallowing, and generalized muscle weakness.
LEMS, on the other hand, is often associated with malignancies, particularly small-cell lung cancer, and results from antibodies targeting the voltage-gated calcium channels on nerve terminals. This impairs the release of acetylcholine, leading to similar but distinct muscle weakness patterns, often beginning in the lower limbs. Both conditions have treatments available, but early diagnosis and management are crucial for optimal outcomes.
Myasthenia Gravis
Myasthenia Gravis (MG) is a chronic autoimmune neuromuscular disease characterized by varying degrees of weakness of the skeletal (voluntary) muscles of the body. The hallmark of myasthenia gravis is muscle weakness that increases during periods of activity and improves after periods of rest. This weakness is caused by a breakdown in the normal communication between nerves and muscles due to antibodies that block, alter, or destroy the receptors for acetylcholine, a neurotransmitter necessary for muscle contraction, at the neuromuscular junction.
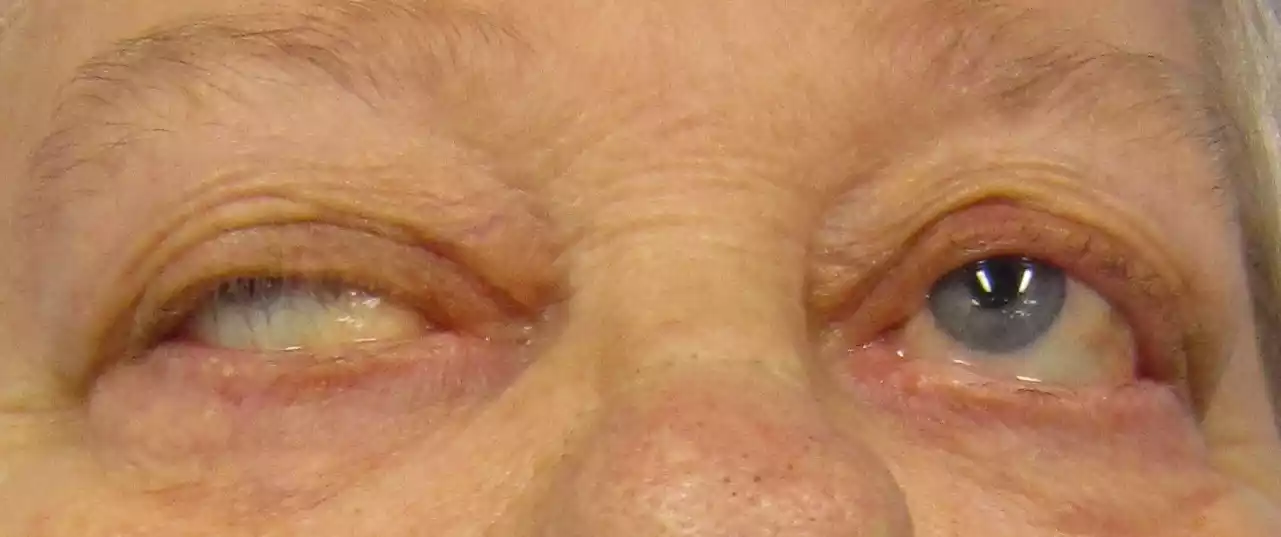
This disruption prevents muscle contraction, leading to muscle weakness. Common symptoms include drooping eyelids, blurred or double vision, slurred speech, difficulty swallowing, and muscle weakness in the arms and legs.
Symptoms of Myasthenia Gravis
Myasthenia Gravis (MG) is characterized by fluctuating muscle weakness and fatigue that worsen with activity and improve with rest. The specific symptoms can vary among individuals, but the following are commonly observed:
- Ocular Symptoms:
- Ptosis: Drooping of one or both eyelids.
- Diplopia: Double vision, which might be horizontal or vertical.
- Bulbar Symptoms:
- Dysphagia: Difficulty swallowing, which can lead to choking or aspiration.
- Dysarthria: Slurred or nasal speech.
- Facial Weakness: Difficulty in making facial expressions like smiling or frowning.
- Generalized Symptoms:
- Muscle Weakness: Particularly in the arms and legs. This may cause difficulty in climbing stairs, lifting objects, or getting up from a sitting position.
- Respiratory Weakness: Weakness in the muscles that assist in breathing can lead to respiratory failure, which is a medical emergency.
- Neck Weakness: Difficulty holding up the head.
- Fatigue: Muscles tire easily, especially after prolonged activity.
- Changes in Voice: A voice that becomes softer or more hoarse.
The severity and combination of these symptoms can vary widely among individuals with MG. Some might only experience eye-related symptoms (ocular MG), while others may have more widespread muscle weakness. In any case, early diagnosis and treatment can help manage the symptoms effectively.
Causes of Myasthenia Gravis
Myasthenia Gravis (MG) is an autoimmune disorder. Its causes are rooted in the immune system’s maladaptive response against the body’s own tissues. Specifically, in MG, the immune system produces antibodies that target and disrupt the neuromuscular junction, where nerve cells communicate with muscles. Here’s a breakdown of the causes:
- Autoantibodies: In most people with MG, the immune system produces antibodies that bind to the acetylcholine receptors on the muscle side of the neuromuscular junction. Acetylcholine is a neurotransmitter, a chemical messenger that transmits signals from nerve cells to muscle cells, leading to muscle contraction. When antibodies block or destroy these receptors, the muscle cells receive fewer signals, resulting in muscle weakness.
- Thymus Gland Abnormalities: The thymus gland, located in the chest beneath the breastbone, is a part of the immune system. It’s believed to play a role in the development of MG. Some MG patients have thymomas (tumors of the thymus, which are usually benign). Even in MG patients without thymomas, the thymus gland is often abnormally large.
- Other Autoantibodies: Though less common, some MG patients have antibodies against other proteins, such as the MuSK protein (muscle-specific kinase) or LRP4 (lipoprotein receptor-related protein 4). These proteins play roles in the neuromuscular junction’s formation and maintenance.
- Genetics: While MG is not directly inherited, there might be a genetic predisposition that makes certain individuals more susceptible to autoimmune disorders.
- External Triggers: Some researchers believe that viruses or other external factors might trigger MG in genetically susceptible individuals, but definitive evidence is still lacking.
While these factors can explain the onset of MG in many patients, the exact cause might remain unclear in some cases. The abnormal immune response leading to MG may result from a combination of genetic and environmental factors.
Identifications of Myasthenia Gravis
The identification (diagnosis) of Myasthenia Gravis (MG) involves a combination of clinical evaluation and specific diagnostic tests. Here’s how MG is typically identified:
- Clinical Evaluation: A detailed history and physical examination is the starting point. The physician will ask about the onset, duration, and pattern of muscle weakness, any exacerbating or relieving factors, and any associated symptoms.
- Edrophonium Test (Tensilon Test): Edrophonium chloride is a drug that temporarily increases the levels of acetylcholine at the neuromuscular junction. When injected, a person with MG will show a marked temporary improvement in muscle strength. However, this test is not used as commonly now due to potential side effects.
- Blood Tests: Blood samples are tested for the presence of acetylcholine receptor antibodies or antibodies against other proteins like MuSK (muscle-specific kinase). A positive test is a strong indicator of MG but not present in all MG patients.
- Electromyography (EMG): A needle electrode is inserted into the muscle to measure the electrical activity. In MG, the response to repetitive nerve stimulation decreases over time, indicating a defect at the neuromuscular junction.
- Single Fiber EMG: This is a specialized form of EMG that measures the electrical activity in individual muscle fibers. It is considered one of the most sensitive tests for MG.
- Pulmonary Function Tests: These are breathing tests to determine the strength of respiratory muscles, especially if there’s concern about respiratory compromise.
- CT or MRI of the Chest: A scan of the chest can detect thymomas or enlargement of the thymus gland, which can be associated with MG.
- Ice Pack Test: This is a non-invasive test sometimes used for patients with ptosis (drooping eyelids). A cold pack is placed on the droopy eyelid for 2 minutes. Improvement in ptosis after removal suggests MG.
- Neostigmine Test: Like the edrophonium test, neostigmine increases acetylcholine at the neuromuscular junction, leading to a transient improvement in muscle strength. However, its effect lasts longer than edrophonium.
It’s essential to remember that no single test can definitively diagnose MG in all patients. Often, a combination of clinical findings and diagnostic tests are used to confirm the diagnosis. Additionally, some of these tests, especially the edrophonium test, have potential side effects, so they are conducted under close medical supervision.
Lambert Eaton Myasthenic Syndrome
Lambert-Eaton Myasthenic Syndrome (LEMS) is a rare autoimmune neuromuscular disorder characterized by muscle weakness and fatigue. It is caused by a decrease in the release of acetylcholine, a neurotransmitter responsible for transmitting nerve impulses to muscle cells, due to antibodies targeting the voltage-gated calcium channels on the presynaptic nerve terminals at the neuromuscular junction.

This disruption leads to impaired nerve signal transmission to muscles, resulting in muscle weakness. LEMS often affects the muscles of the legs first, and it can be associated with other medical conditions, particularly small cell lung cancer. Symptoms may also include dry mouth, impotence in males, and changes in vision. Unlike myasthenia gravis, muscle strength in LEMS can temporarily improve with repeated use but may fatigue again with continuous activity.
Symptoms of Lambert Eaton Myasthenic Syndrome
Lambert-Eaton Myasthenic Syndrome (LEMS) is a rare autoimmune disorder that affects the neuromuscular junction, leading to muscle weakness and other symptoms. Here are the common symptoms associated with LEMS:
- Muscle Weakness:
- Proximal Muscle Weakness: The muscles closest to the center of the body, especially those in the hips and shoulders, are affected first and most severely.
- Leg Weakness: Difficulty climbing stairs or getting up from a seated position.
- Arm Weakness: Difficulty lifting objects.
- Autonomic Symptoms:
- Dry Mouth: Due to reduced secretion of saliva.
- Impotence: In men, there can be difficulty achieving or maintaining an erection.
- Constipation: Reduced bowel movements.
- Blurred Vision: Due to disturbance in the autonomic nerves affecting the eye.
- Reduced Sweating: Leading to heat intolerance.
- Muscle Fatigability: Unlike Myasthenia Gravis where symptoms worsen with sustained activity, in LEMS, muscle strength might improve with repeated use, but then deteriorates again with continuous activity.
- Ocular Symptoms:
- Ptosis: Drooping of one or both eyelids, though this is less common than in Myasthenia Gravis.
- Diplopia: Double vision.
- Difficulty Swallowing (Dysphagia): This can lead to choking or aspiration.
- Difficulty Speaking (Dysarthria): Speech may be soft or nasal.
- Facial Weakness: Difficulty in making facial expressions.
- Respiratory Weakness: While less common, weakness in the muscles that assist in breathing can be serious, leading to respiratory failure in severe cases.
One important distinguishing factor about LEMS is its association with malignancies. Approximately 50-60% of individuals with LEMS have an underlying malignancy, most commonly small cell lung cancer. Therefore, the diagnosis of LEMS often warrants a thorough search for an associated tumor.
The symptoms of LEMS can be similar to other neuromuscular disorders, particularly Myasthenia Gravis, so proper diagnostic tests and clinical evaluations are crucial for accurate diagnosis and management.
Causes of Lambert Eaton Myasthenic Syndrome
Lambert-Eaton Myasthenic Syndrome (LEMS) is primarily caused by an autoimmune response. The body’s immune system mistakenly targets and disrupts the normal function of the neuromuscular junction. Here’s a more detailed look into the causes:
- Autoantibodies Against Voltage-Gated Calcium Channels (VGCCs):
- The primary pathological feature of LEMS is the presence of autoantibodies against VGCCs on the presynaptic nerve terminals at the neuromuscular junction.
- VGCCs play a crucial role in the release of acetylcholine, a neurotransmitter responsible for muscle contraction. The binding of these autoantibodies reduces the number of functional VGCCs, leading to a decreased release of acetylcholine and subsequent muscle weakness.
- Association with Malignancies:
- Paraneoplastic LEMS: Approximately 50-60% of LEMS cases are associated with an underlying malignancy, especially small cell lung cancer (SCLC). In these cases, the body’s immune response to the tumor cross-reacts with VGCCs, leading to the symptoms of LEMS.
- It’s believed that the tumor cells express proteins similar to those of VGCCs, causing the immune system to inadvertently attack both the tumor and the neuromuscular junction.
- Idiopathic LEMS:
- In some cases, LEMS can occur without a detectable tumor or clear cause. This form is termed idiopathic LEMS. While the autoimmune response against VGCCs is still present, the triggering event or reason for this response is unclear.
- Possible Triggers:
- Besides tumors, other potential triggers for the autoimmune response in LEMS might include viral infections, though this is not definitively established.
- Other Autoantibodies:
- Though less common, some patients with LEMS might have antibodies against other components of the neuromuscular junction, such as SOX1, which is especially linked to LEMS with SCLC.
The strong association of LEMS with malignancies, as the identification and treatment of an underlying tumor can lead to an improvement in neurological symptoms. Regular cancer screenings are recommended for individuals diagnosed with LEMS to monitor for potential malignancies.
Identifications of Lambert Eaton Myasthenic Syndrome
The identification (diagnosis) of Lambert-Eaton Myasthenic Syndrome (LEMS) involves a combination of clinical evaluation and specific diagnostic tests. Here’s how LEMS is typically identified:
- Clinical Evaluation: The doctor will take a thorough medical history, asking about the onset, pattern, and duration of muscle weakness and other associated symptoms.
- Electromyography (EMG) and Nerve Conduction Studies:
- Repetitive Nerve Stimulation: In patients with LEMS, there’s typically a decremental response in muscle action potentials with low-frequency (2-3 Hz) stimulation, but an incremental response (often greater than 100%) with high-frequency (20-50 Hz) stimulation.
- Single Fiber EMG: This can show increased jitter and blocking, reflecting the disordered neuromuscular transmission characteristic of LEMS.
- Blood Tests:
- Serum can be tested for the presence of antibodies against voltage-gated calcium channels (VGCCs), a hallmark of LEMS. However, not all patients with LEMS test positive for these antibodies.
- Other autoantibodies, such as those against SOX1, might also be checked, especially if there’s suspicion of an associated small cell lung cancer.
- CT or PET Scan of the Chest: Given the strong association between LEMS and small cell lung cancer, imaging studies of the chest are essential to rule out an underlying malignancy.
- Autonomic Function Tests: Since LEMS can affect autonomic functions, tests that measure sweating, heart rate, and blood pressure changes can be useful in some cases.
- Response to Treatment: Some patients might show a marked improvement in muscle strength after receiving an intravenous injection of guanidine hydrochloride or 3,4-diaminopyridine, which can support the diagnosis.
- Differential Diagnosis: It’s essential to differentiate LEMS from other neuromuscular and autoimmune disorders, especially Myasthenia Gravis (MG). Certain features and tests can help distinguish between these conditions.
Regular screenings for cancer are crucial in LEMS patients, given the potential association with malignancies, especially small cell lung cancer. Proper identification is crucial not only for appropriate treatment of LEMS but also for the management of any associated conditions.
Comparison table of Myasthenia Gravis and Lambert Eaton Myasthenic Syndrome
Here’s a comparison table of Myasthenia Gravis (MG) and Lambert-Eaton Myasthenic Syndrome (LEMS):
| Feature | Myasthenia Gravis (MG) | Lambert-Eaton Myasthenic Syndrome (LEMS) |
|---|---|---|
| Etiology | Autoimmune attack on acetylcholine receptors | Autoimmune attack on voltage-gated calcium channels |
| Association with Cancer | Thymoma (tumor of the thymus) is common | Strongly associated with small cell lung cancer |
| Primary Muscles Affected First | Ocular (e.g., eyelids) | Proximal muscles, especially in the legs |
| Muscle Strength with Use | Worsens with continued activity | Can improve with repeated use but fatigues with sustained activity |
| Common Symptoms | Drooping eyelids, double vision, difficulty swallowing, generalized weakness | Leg weakness, dry mouth, impotence, changes in vision |
| Diagnostic Tests | Edrophonium (Tensilon) test, antibodies to acetylcholine receptor | Electromyography (EMG), antibodies to voltage-gated calcium channels |
| Treatment | Acetylcholinesterase inhibitors (e.g., pyridostigmine), immunosuppressants, thymectomy | Diaminopyridine, immunosuppressants, treatment of underlying cancer (if present) |
This table provides a summarized comparison between the two conditions, but both MG and LEMS have their own intricacies and variations. It’s essential to understand that while they share some similarities, they are distinct conditions with different management approaches.
Treatment Approaches for Myasthenia Gravis and LEMS
Both Myasthenia Gravis (MG) and Lambert-Eaton Myasthenic Syndrome (LEMS) are autoimmune disorders that affect the neuromuscular junction, but they have distinct pathophysiologies and therefore, some differences in treatment.

Below is a comparison of the treatment approaches for MG and LEMS:
Myasthenia Gravis (MG) Treatment Approaches:
- Anticholinesterase Medications: Pyridostigmine (Mestinon) is used to enhance neuromuscular transmission by inhibiting acetylcholine breakdown.
- Corticosteroids: Prednisone or other corticosteroids suppress the immune system and decrease the production of harmful antibodies.
- Immunosuppressant Drugs: Azathioprine (Imuran), Mycophenolate mofetil (CellCept), and Cyclosporine are used to suppress the immune system, reducing the production of harmful antibodies.
- Thymectomy: Surgical removal of the thymus gland benefits many MG patients, especially if they have thymoma.
- Plasmapheresis and IVIG: These therapies can be used in acute exacerbations or as bridging therapies before surgery or other treatments.
- Monoclonal Antibodies: Eculizumab (Soliris) targets the complement system and is used for adults with AChR-positive generalized MG.
Lambert-Eaton Myasthenic Syndrome (LEMS) Treatment Approaches:
- Potassium Channel Blockers: 3,4-diaminopyridine (3,4-DAP) increases the release of acetylcholine from nerve endings and is a first-line treatment for LEMS.
- Immunosuppressant Drugs: Prednisone, Azathioprine, and other immunosuppressive drugs can be used in LEMS, similar to MG.
- Plasmapheresis and IVIG: While less commonly used in LEMS than in MG, these can be effective, especially in acute exacerbations or when rapid symptom improvement is needed.
- Treatment of Underlying Malignancy: Since LEMS is often associated with small cell lung cancer, treating the tumor can result in an improvement in LEMS symptoms. This might involve chemotherapy, radiation, or surgery.
- Monoclonal Antibodies: Rituximab targets B-cells and has shown promise in some LEMS cases, especially when other treatments are ineffective or contraindicated.
Shared Aspects for Both Conditions:
- Avoidance of Exacerbating Medications: Certain medications can exacerbate symptoms in both MG and LEMS, so it’s crucial for patients to be informed and avoid these drugs.
- Regular Monitoring: Patients should have regular neurological assessments, and sometimes, repeated diagnostic tests to monitor disease progression and response to treatments.
- Physical and Occupational Therapy: Both conditions can benefit from physical rehabilitation and adaptive strategies.
- Support and Education: Support groups, patient education, and counseling can help patients cope with their condition and stay informed.
The best treatment approach depends on the individual’s specific diagnosis, the severity of symptoms, associated conditions, and other personal factors. A multidisciplinary approach often ensures the best outcomes for patients with these neuromuscular disorders.
How to prevent Myasthenia Gravis and LEMS
As of my last update in January 2022, there is no known method to directly prevent the onset of Myasthenia Gravis (MG) or Lambert-Eaton Myasthenic Syndrome (LEMS), as both are autoimmune diseases with multifactorial causes that are not fully understood. While you cannot directly prevent these diseases, there are general measures to reduce the risk of autoimmune disorders and strategies to prevent exacerbations or complications in people who are already diagnosed:
- General Autoimmune Prevention Strategies:
- Maintain a Healthy Lifestyle: Regular exercise, a balanced diet, and stress management can potentially bolster the immune system and reduce the risk of autoimmune conditions.
- Avoid Environmental Triggers: In some autoimmune diseases, environmental toxins or certain medications might trigger or exacerbate symptoms. Being informed about potential triggers and avoiding them can be beneficial.
- Regular Health Check-ups: Regular medical check-ups can detect early signs of autoimmune diseases, potentially allowing for early intervention.
- Specific to LEMS:
- Cancer Screening: Given the association between LEMS and small cell lung cancer, regular screenings can help in early detection and treatment of the underlying malignancy. Addressing the malignancy can often lead to an improvement in LEMS symptoms.
- For Those Diagnosed:
- Avoid Medications that Exacerbate Symptoms: Some medications can worsen the symptoms of MG and LEMS. Always consult with healthcare providers when starting new medications.
- Monitor for Infections: Infections can trigger exacerbations. Maintaining good hygiene, getting vaccinated, and promptly treating infections can help.
- Thymectomy in MG: If a patient with MG has a thymoma or is a young adult with generalized MG, thymectomy might be recommended. This procedure can reduce the severity of MG and might prevent some of its complications.
- Regular Monitoring: Regular visits to a neurologist or specialist can ensure that the disease is optimally managed, and complications are prevented or promptly addressed.
- Patient Education: Being well-informed about the disease can help patients recognize signs of exacerbations early and take steps to manage their condition effectively.
While you might not be able to prevent MG or LEMS, early detection and appropriate management can significantly improve quality of life and outcomes. It’s always a good idea to seek updated information and advice from healthcare professionals regarding prevention and management strategies.
Conclusion
Myasthenia Gravis (MG) and Lambert-Eaton Myasthenic Syndrome (LEMS) are both autoimmune neuromuscular disorders characterized by muscle weakness. MG primarily results from antibodies that block or destroy nicotinic acetylcholine receptors at the neuromuscular junction, leading to decreased muscle activation.
LEMS, on the other hand, is often associated with an underlying malignancy and involves autoantibodies targeting voltage-gated calcium channels, reducing acetylcholine release. While both conditions share some therapeutic strategies, their distinct pathophysiological mechanisms necessitate tailored treatment approaches. Early diagnosis and a multidisciplinary management approach are crucial for optimal patient outcomes.